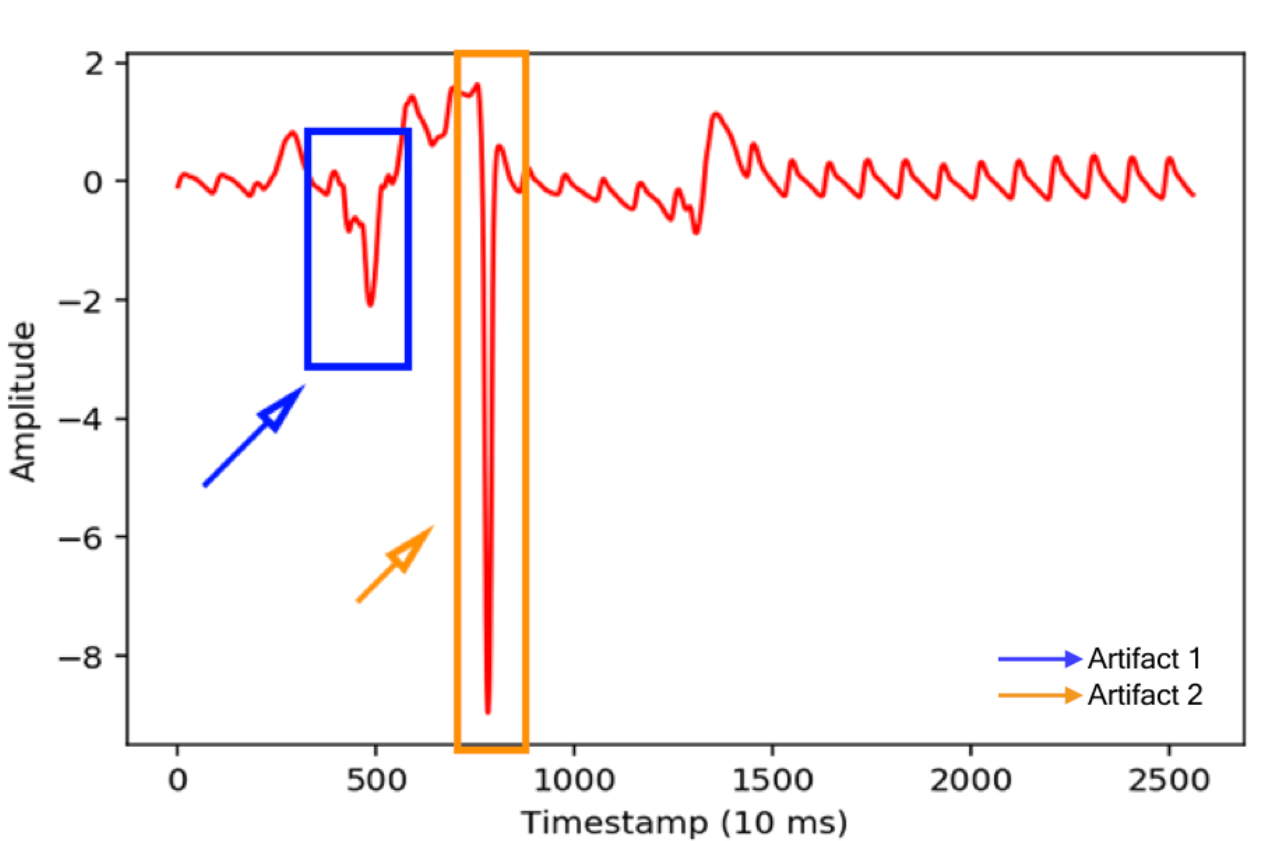
Read the Full PapeR @ nature medicine
Robert Avram, Jeffrey E. Olgin, Peter Kuhar, J. Weston Hughes, Gregory M Marcus, Mark J Pletcher, Kirstin Aschbacher*, Geoffrey H Tison*
Tison Lab | The Health eHeart Study
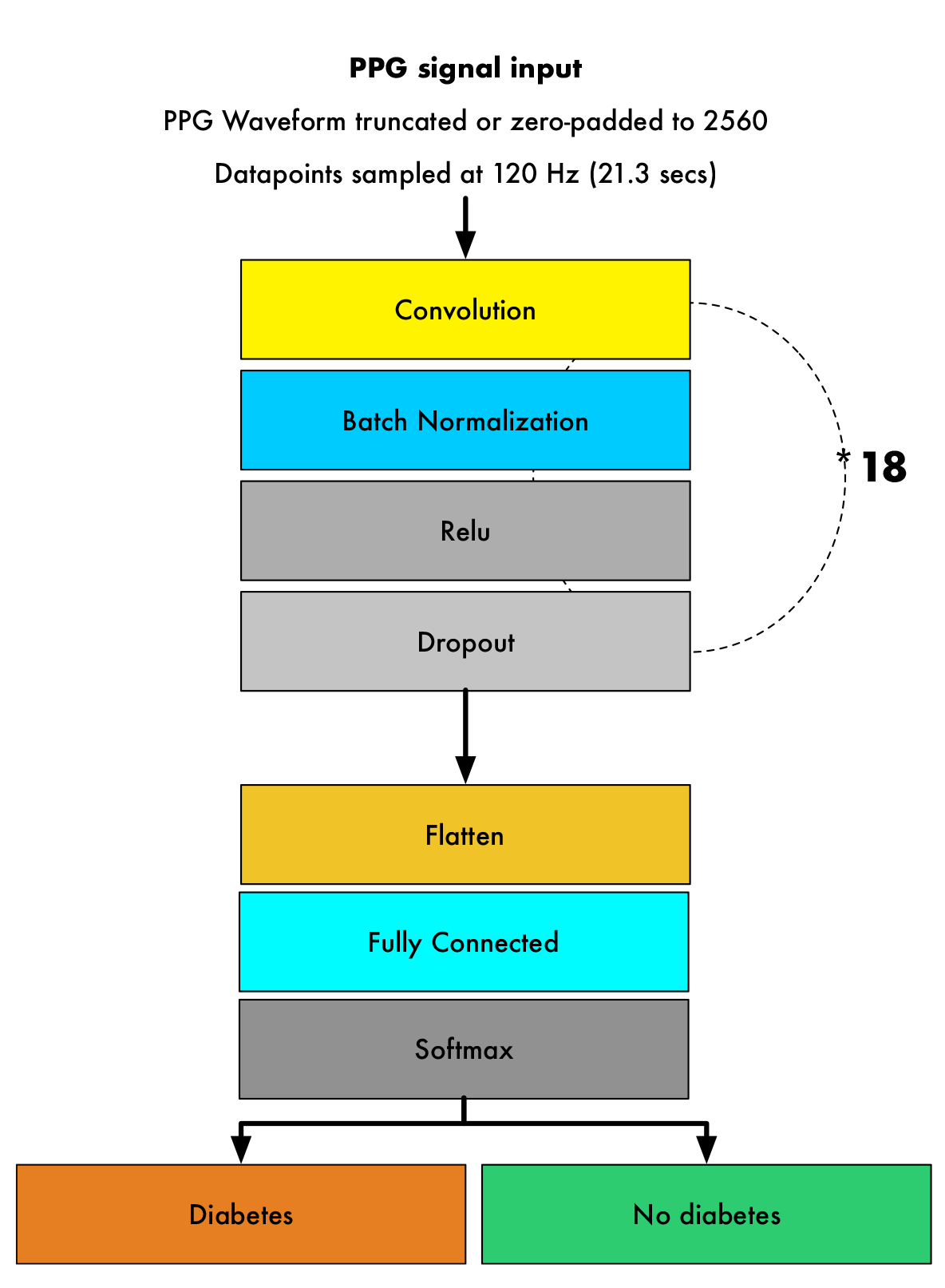
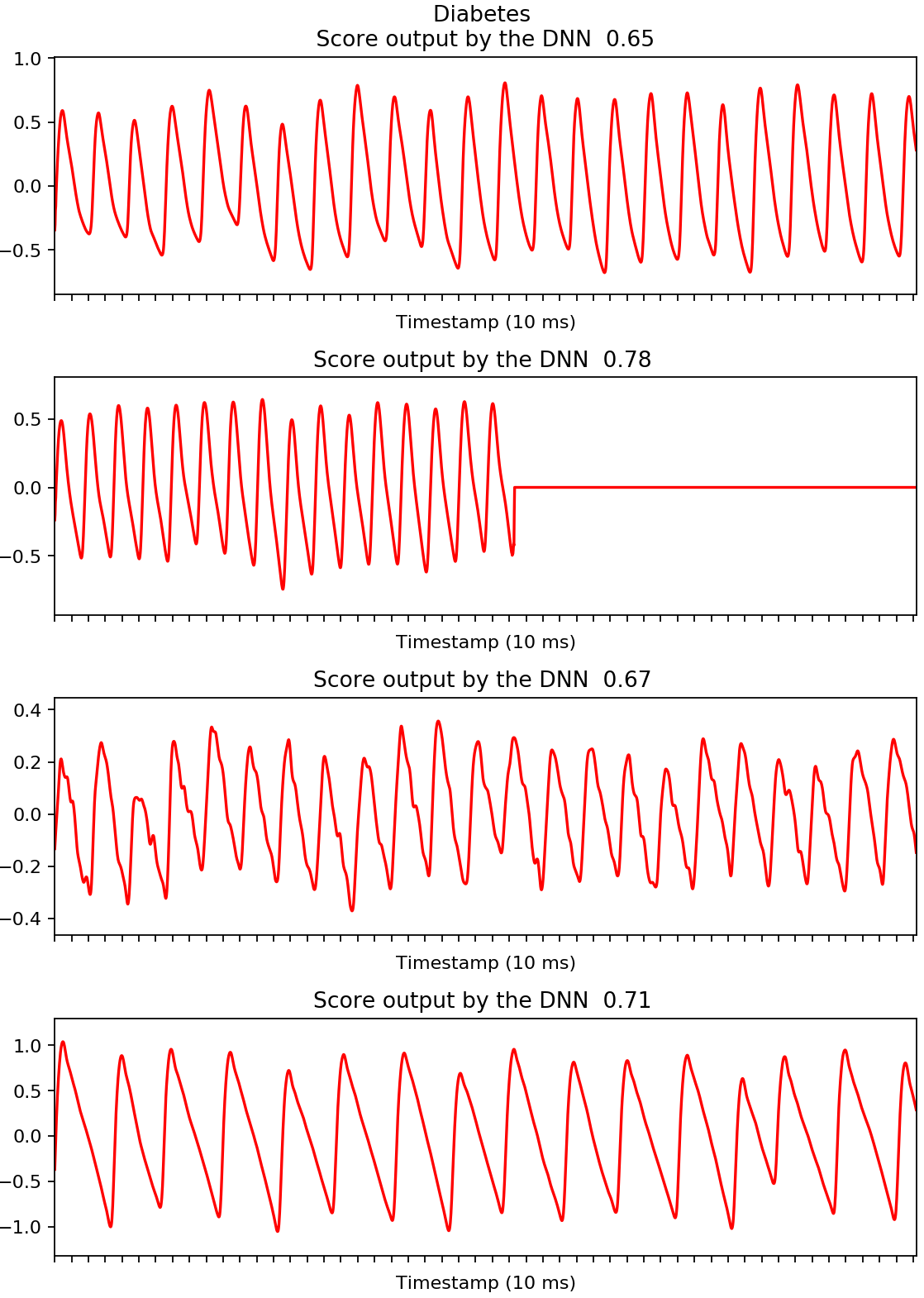
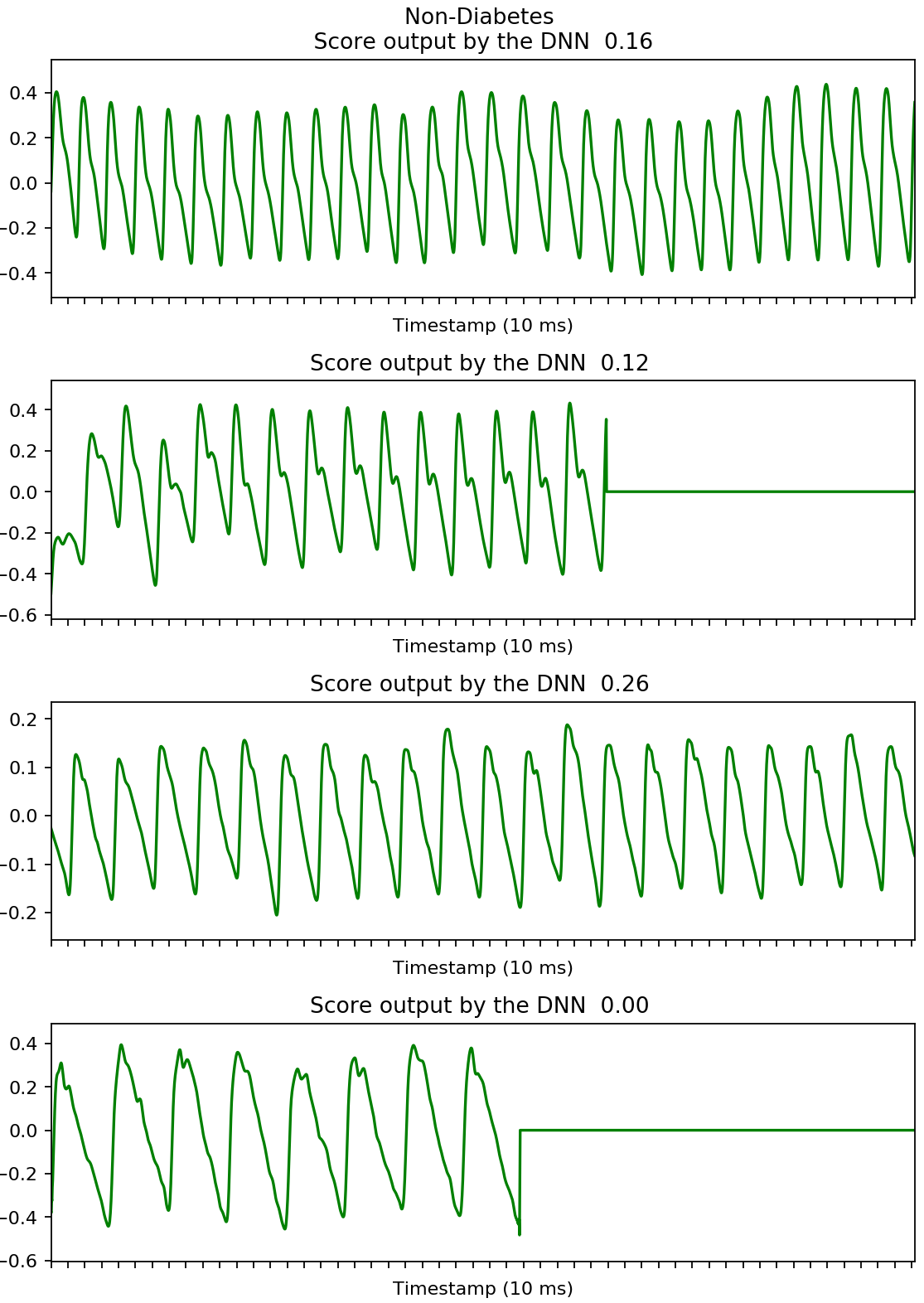
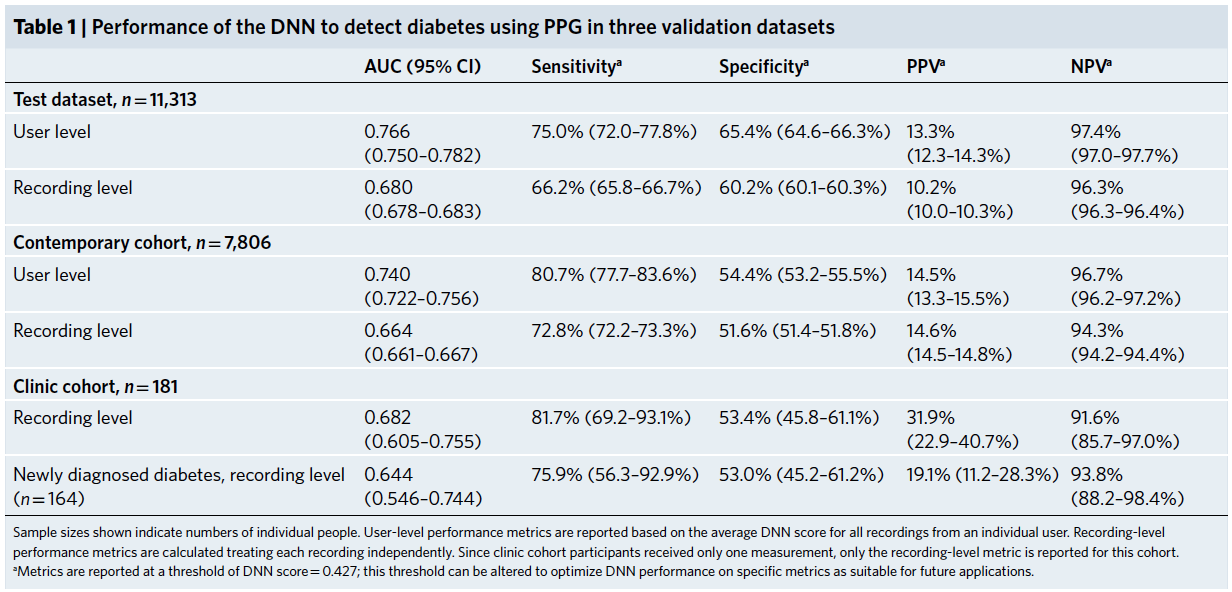
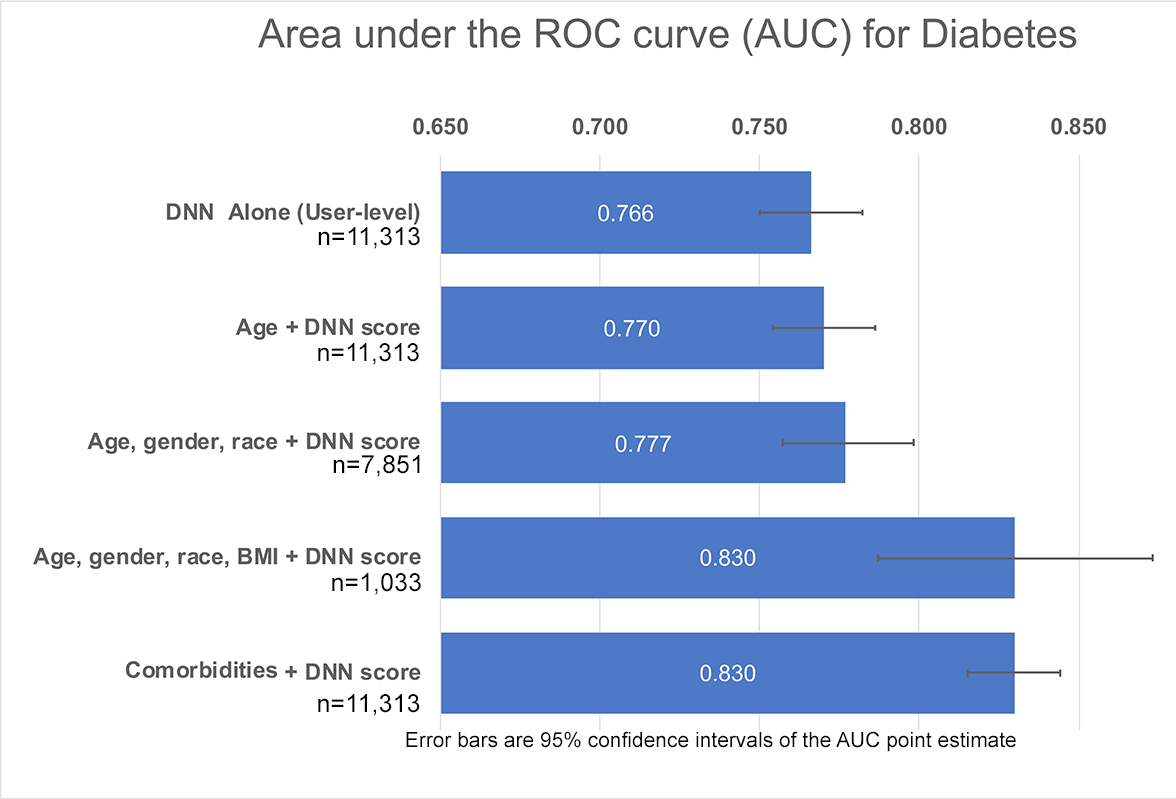
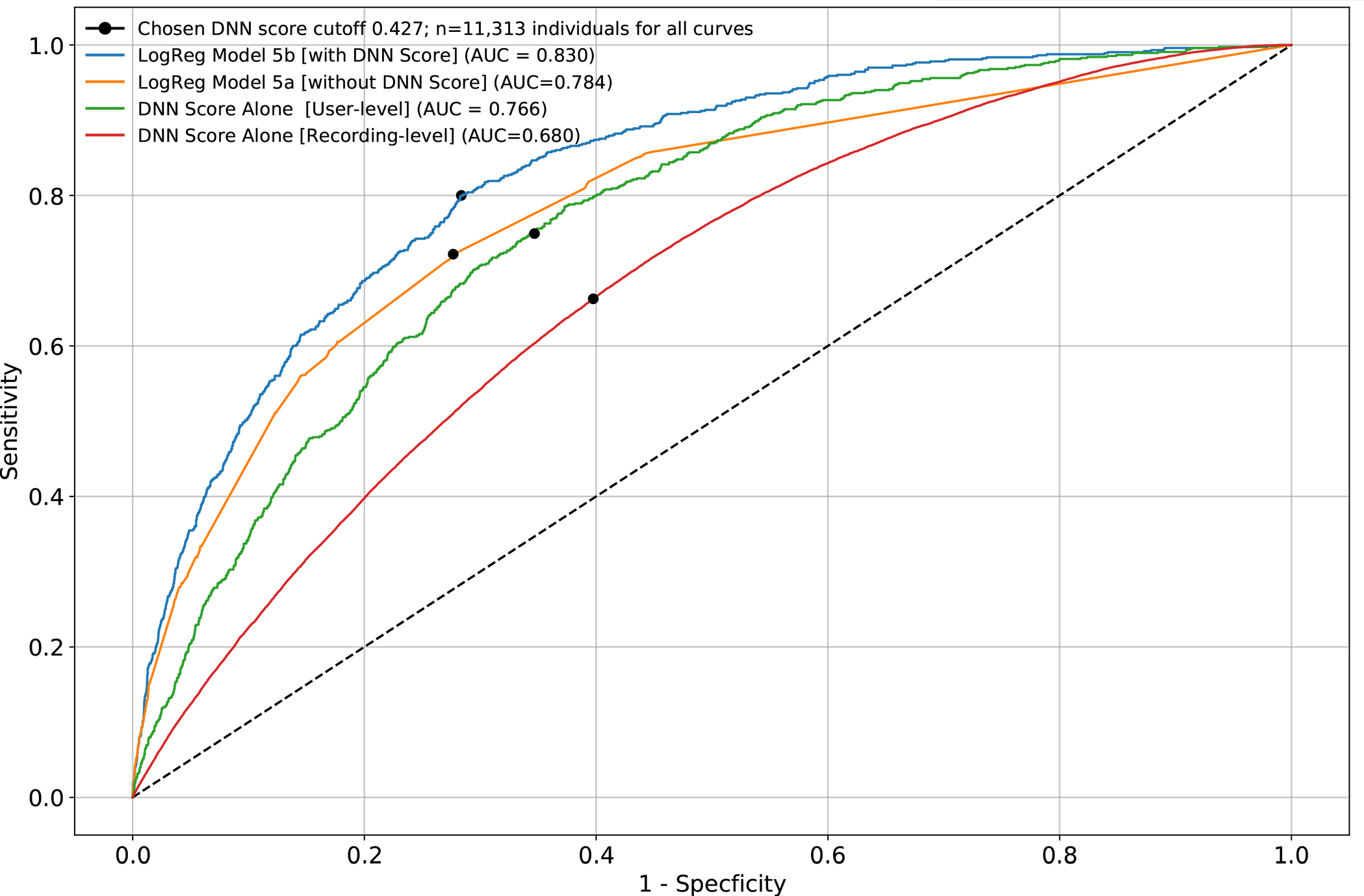
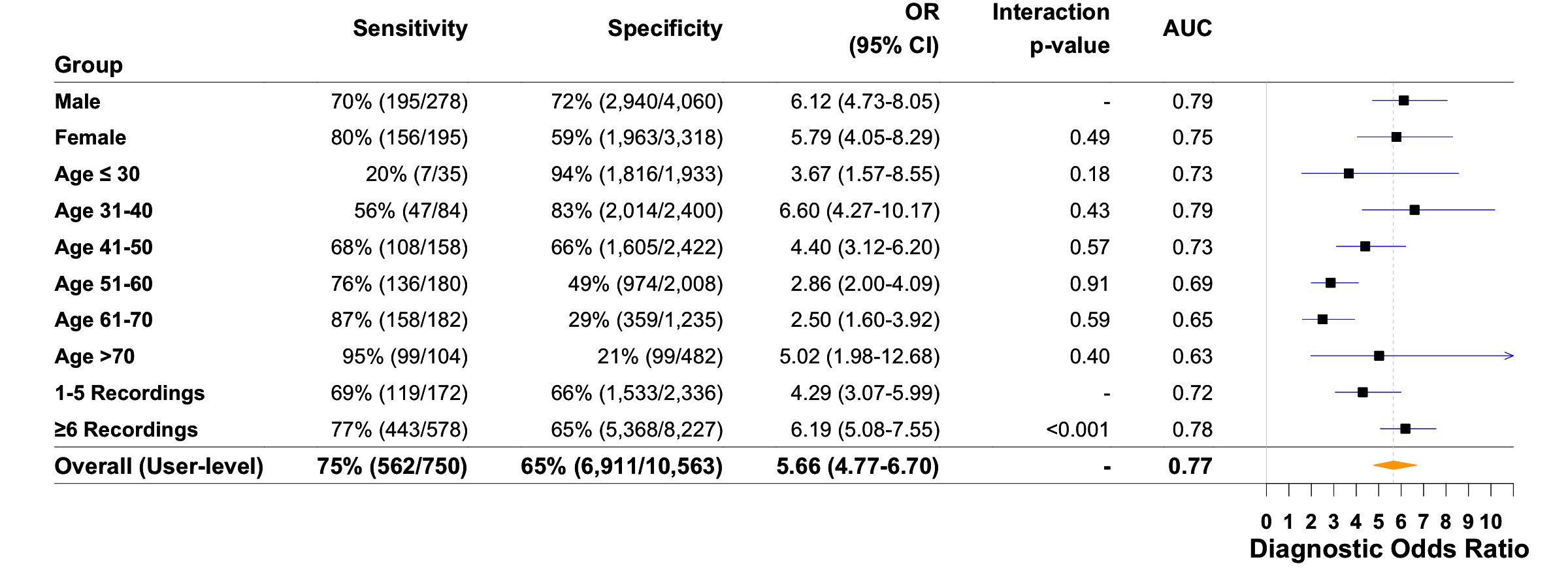
We developed a deep neural network algorithm to detect diabetes from vascular signals, called PPG or photoplethysmography, that can be measured with existing hardware in smart devices like smartphones and fitness trackers. This is the same technology that many wearable devices and hospitals use to measure heart rate.